- Folder Science News
- Views 126
- Last Updated 29/01/2026
Recently, several infant formula products have been recalled due to potential contamination with cereulide. The toxin produced by Bacillus cereus is associated with nausea and vomiting. In vulnerable populations, including infants and immunocompromised individuals, exposure may result in severe intoxication. In light of current scientific evidence, the Vietnam Center for Food Safety Risk Assessment of the National Institute for Food Control has compiled and summarized key information regarding the causative microorganism and the toxin involved.
Food poisoning associated with Bacillus cereus
Bacillus cereus is a Gram-positive, spore-forming bacterium that can grow over a temperature range of 5-50°C (optimal growth at 35-40°C) and across a pH range of 4.5-9.3. Owing to the durability and persistence of its spores in the environment, this microorganism is capable of contaminating numerous food matrices. In the field of food safety, B. cereus is recognized as a cause of foodborne illness, primarily associated with the production of two distinct toxin types: the emetic toxin and the diarrheal toxin.
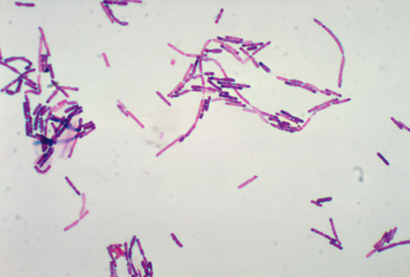
Figure 1. Microscopic morphology of B. cereus
Food groups that have been reported to be at high risk of contamination with this bacterium include milk and dairy products, meat and meat products, vegetables, spice mixtures, dried foods, as well as cereal-based products such as bread, cereal grains, and pizza.
Emetic toxin cereulide
Cereulide is the emetic toxin produced by certain strains of B. cereus during bacterial growth. This toxin is colorless, odorless, and tasteless. It has a cyclic peptide structure, which renders it resistant to degradation by conventional cooking temperatures as well as to inactivation by digestive enzymes after ingestion. Consequently, the safety of food products cannot be assessed solely based on normal heat treatment.
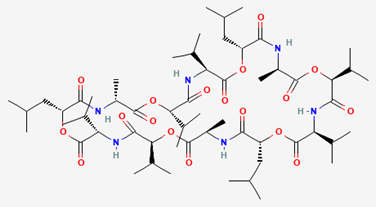
Figure 2. Structure of cereulide
Cereulide-related food poisoning results from the ingestion of preformed toxin present in food before consumption, rather than from toxin production within the human body after ingestion. Toxin formation may take place when raw materials or food products are contaminated with B. cereus and exposed to conditions favorable for bacterial growth. Recent investigations in Europe have suggested that the occurrence of cereulide in certain infant nutrition products may be linked to specific raw materials, notably arachidonic acid oil (ARA oil) incorporated during manufacturing. This observation provides a scientific basis for the detection of cereulide in processed foods despite the absence of viable B. cereus, thereby highlighting the necessity for thorough and precautionary risk assessment.
Food poisoning caused by cereulide
Cereulide intoxication is characterized by a rapid onset, with symptoms typically occurring 30 minutes to 6 hours after consumption of contaminated food and generally resolving within 24 hours. The most common clinical symptoms include nausea, vomiting, and moderate gastrointestinal discomfort. Nevertheless, severe toxic effects have been reported in certain cases, including hepatic, intestinal, and pancreatic dysfunction or impairment. Vulnerable populations, such as infants and immunocompromised individuals, are at a higher risk of developing complications.
At present, the Codex Alimentarius Commission and most countries have not established regulatory limits for cereulide in infant formula or other food products. The lack of established limit values for cereulide primarily reflects fragmented and non-standardized data from diverse sources and study models, which are insufficient to define harmonized human safety thresholds. However, available evidence from animal studies suggests that the minimum intoxication dose of cereulide is approximately 8-10 µg/kg body weight. Despite the absence of formal limits, the European Union Rapid Alert System for Food and Feed (RASFF) has reported and issued alerts for powdered infant formula products when cereulide was detected at concentrations ranging from 0.4 to 2.7 µg/kg, highlighting a significant concern for food safety.
On 28 January 2026, the European Food Safety Authority (EFSA) was requested by the European Commission to conduct a risk assessment of this toxin. The assessment is expected to provide key outputs, including the acute reference dose (ARfD), safety thresholds, and risk management measures specifically addressing infant populations.
To date, no confirmed cases of food poisoning associated with the consumption of infant formula have been reported in Vietnam. The Ministry of Health has issued an official directive to provincial Departments of Health and local Food Safety Administration to conduct reviews and implement preventive measures in order to mitigate potential risks to consumer health arising from infant formula products currently marketed in Vietnam.
Analysis of cereulide in food
The detection of cereulide requires advanced analytical approaches, such as mass spectrometry-based chromatographic techniques or specific biological methods. Currently, the National Institute for Food Control is conducting studies to determine cereulide in infant formula using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in accordance with ISO 18465:2017. In parallel, the Vietnam Center for Food Safety Risk Assessment is continuously updating scientific evidence related to B. cereus and cereulide in order to provide science-based advice for risk management and risk communication concerning this toxin.
Written by: Nguyen Tuan Thanh Vietnam Center for Food Safety Risk Assessment
Pham Ngoc Ha, Laboratory of Food Microbiology and Genetically modified food
References
1. Berthold-Pluta A, Pluta A, Garbowska M, Stefańska I. Prevalence and Toxicity Characterization of Bacillus cereus in Food Products from Poland. Foods. 2019;8(7).
2. Yang S, Wang Y, Liu Y, Jia K, Zhang Z, Dong Q. Cereulide and Emetic Bacillus cereus: Characterizations, Impacts and Public Precautions. Foods. 2023;12(4):833.
3. Kranzler M, Stollewerk K, Rouzeau-Szynalski K, Blayo L, Sulyok M, Ehling-Schulz M. Temperature Exerts Control of Bacillus cereus Emetic Toxin Production on Post-transcriptional Levels. Front Microbiol. 2016;7:1640.
4. Bacillus cereus in food: when it multiplies, the bacterium can produce a toxin - BfR. Accessed: Jan. 28, 2026. Available: https://www.bfr.bund.de/en/notification/bacillus-cereus-in-food-when-it-multiplies-the-bacterium-can-produce-a-toxin/
5. R. Vangoitsenhoven, M. Maris, L. Overbergh, J. Van Loco, C. Mathieu, and B. Van der Schueren. Cereulide food toxin, beta cell function, and diabetes: Facts and hypotheses. Diabetes Res. Clin. Pract. 2015; vol. 109, no. 1, pp. 1-5.















