- Folder Technical News
- Views 2317
- Last Updated 02/01/2025
Dietary fiber has been scientifically proven to provide significant health benefits and is considered an essential component of the daily diet. Given the diversity of fiber compositions in various food products, selecting an appropriate analytical method to assess dietary fiber is crucial for ensuring product quality, optimizing time efficiency, and reducing analytical costs.
1. Definition of Dietary Fiber
According to the Codex Alimentarius Commission (CAC) [1], dietary fibre refers to carbohydrate polymers consisting of three or more monomeric units that are not hydrolysed by endogenous enzymes in the human small intestine. Dietary fibres fall into the following categories:
- Naturally occurring carbohydrate polymers found in food products.
- Carbohydrate polymers extracted from food raw materials through physical, enzymatic, or chemical methods.
- Synthetic carbohydrate polymers.
2. Classification of Dietary Fiber
Based on water solubility, dietary fibre can be classified into two main categories: soluble fibre and insoluble fibre [2].
Soluble Fiber: This type of fibre dissolves in water, forming a gel-like substance in the digestive tract. Soluble fibres include fructans (inulin, fructo-oligosaccharides (FOS)), pectin, β-glucan, galactomannan, glucomannan, polydextrose, psyllium, galacto-oligosaccharides (GOS), and dextrin. These fibres are commonly found in oats, barley, fruits, and vegetables such as apples, oranges, carrots, and legumes (e.g., soybeans, kidney beans, black beans).
Insoluble Fiber: This type of fibre does not dissolve in water, does not form gels, and resists fermentation in the colon by gut microbiota. It includes components such as cellulose, hemicelluloses, lignin, resistant starch, arabinoxylan, cutin, suberin, and non-starch polysaccharides. Insoluble fibres are present in wheat bran, whole grains, corn, cauliflower, green beans, and potatoes.
3. Roles of Dietary Fiber in Human Health
Dietary fibre plays a crucial role in maintaining overall health, including supporting digestion, preventing constipation, promoting cardiovascular health, regulating blood sugar levels, aiding weight management, and reducing the risk of colorectal and breast cancers [3].
Digestive Support and Prevention of Constipation
Dietary fibre absorbs water, increases the bulk of intestinal contents, and softens stool, facilitating regular bowel movements and preventing constipation. Additionally, fibre regulates gut microbiota by fostering the growth of beneficial bacteria, enhancing digestive and absorptive processes in the intestines.
Cardiovascular Health
Elevated cholesterol levels are a primary contributor to cardiovascular diseases. Fermentation of dietary fibre by gut microbiota inhibits cholesterol synthesis in the liver and produces short-chain fatty acids (SCFAs) that reduce serum cholesterol by lowering low-density lipoprotein (LDL) levels and increasing high-density lipoprotein (HDL) levels. This mechanism mitigates the risks of hyperlipidemia, coronary artery disease, and atherosclerosis.
Blood Sugar Regulation
Dietary fibre prolongs gastric emptying time and slows the digestion and absorption of glucose, leading to gradual blood sugar increases. For individuals with diabetes, consuming soluble fibre-rich foods can aid in controlling blood glucose levels and reducing insulin requirements. Studies have demonstrated that incorporating one to two servings of beans, oats, psyllium, or other soluble fibre sources into the diet can significantly lower fasting blood glucose levels.
Weight Management
Foods rich in dietary fibre are typically low in calories, sugar, and fat, making them ideal for health-conscious individuals. Fiber-rich foods require longer chewing times, promote a feeling of fullness, and sustain satiety for extended periods, thereby reducing appetite and preventing overeating. Consequently, a diet rich in dietary fibre is a recommended strategy for weight control.
Cancer Prevention
Research has highlighted the role of dietary fibre in reducing the risk of colorectal cancer by supporting beneficial gut bacteria, which produce substances that inhibit cancer cell proliferation and facilitate the excretion of carcinogens. Additionally, dietary fibre reduces the transit time of waste materials through the colon, minimizing exposure to carcinogenic substances. In breast cancer prevention, dietary fibre has been shown to increase the excretion of estrogen in feces and reduce its reabsorption in the intestines, resulting in lower plasma estrogen levels. Fiber also contributes to mitigating insulin resistance and insulin-like growth factor activity, both of which are implicated in breast cancer development [4].
The recommended daily intake of dietary fibre for Vietnamese adults is 20–22 grams per person. However, the average dietary fibre consumption in Vietnam is estimated at only 10 grams per day. To address this deficit, it is essential to not only increase the consumption of fibre-rich natural foods but also incorporate supplemental dietary fibres into functional foods. Common supplemental fibres include fructans (inulin, FOS), GOS, polydextrose, resistant starch, and β-glucan, extracted from various natural sources and processed into diverse forms (e.g., tablets, capsules, syrups, solutions, and powders). Given the variability in the quality of fibre-containing products due to differences in sourcing and production methods, rigorous analytical testing of dietary fibre content is imperative. Such evaluations ensure product quality, safeguard consumer rights, and maintain confidence in the functional food market.
4. Analytical Methods for Dietary Fiber in Food Products
Currently, dietary fiber analysis techniques are continuously updated and revised by AOAC to enable comprehensive evaluation of fiber components in food products. At the National Institute for Food Control (NIFC), various methods have been employed to quantify specific dietary fibers as well as total dietary fiber content.
Analysis of Specific Dietary Fiber Components
- Fructans
Fructans are fructose polymers that may contain glucose and are categorized as inulin (10–60 fructose units) or fructo-oligosaccharides (FOS, 3–10 fructose units) based on chain length. Fructan quantification involves enzymatic hydrolysis. Fructans are extracted using hot water, while non-fructan components (e.g., sucrose, lactose, and starch) are hydrolysed into monosaccharides and converted to polyols using alkaline borohydride. Fructans are then hydrolyzed by fructanase into fructose and glucose, which can be analysed by ion-exchange chromatography or spectrophotometry (derivatization with p-hydroxybenzoic acid hydrazide). Depending on the sample matrix, methods such as AOAC 997.08, AOAC 999.03, AOAC 2016.14, or AOAC 2018.07 are employed.
- Galacto-Oligosaccharides (GOS)
GOS consist of galactose units linked to lactose, serving as a prebiotic for beneficial gut microbiota. Quantification involves enzymatic hydrolysis. GOS is extracted with phosphate buffer at 80°C, followed by enzymatic hydrolysis of non-GOS components using amyloglucosidase. GOS is hydrolysed by β-galactosidase into galactose and lactose, which are analysed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) under AOAC 2001.02.
- Polydextrose
Polydextrose, a polymer synthesized from glucose molecules, is widely utilized in bakery products, confectionery, and beverages to enhance moisture retention, stabilization, and sweetness. Quantification of polydextrose in food products is performed using an enzymatic method. The analyte is extracted with hot water, centrifuged, and the supernatant is filtered through an ultrafiltration membrane to eliminate high-molecular-weight interferents. The filtrate undergoes enzymatic treatment with a mixture of isoamylase, amyloglucosidase, and fructanase to remove oligosaccharides (e.g., malto-oligomers and fructans). Subsequently, the treated sample and a polydextrose standard are analyzed using high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD).
- Resistant starch (RS)
RS refers to starch fractions that escape enzymatic digestion in the human small intestine. Non-resistant starch is hydrolyzed into glucose using a combination of α-amylase and amyloglucosidase (AMG) during a 16-hour incubation at 37°C. Resistant starch is isolated as a pellet after centrifugation, then solubilized in potassium hydroxide, neutralized with acetate buffer, and further hydrolyzed into glucose by AMG. The liberated glucose is quantified using the glucose oxidase-peroxidase (GOPOD) method according to AOAC 2002.02.
- β-Glucan
β-Glucan is a soluble fiber composed of D-glucose molecules linked by β-glycosidic bonds and is a natural component of bacterial, fungal, and cereal cell walls (e.g., oats and barley). Its quantification follows AOAC 995.16, which targets mixed-linkage (1-3, 1-4)-β-D-glucan. Sample extraction is carried out in a buffer solution (pH 6.5), followed by enzymatic hydrolysis using lichenase and β-glucosidase to convert β-glucan into β-D-glucose. The resulting glucose undergoes oxidation with glucose oxidase/peroxidase, and the absorbance of the colored reaction product is measured at 510 nm for β-glucan quantification.
Total Dietary Fiber Analysis
The quantification of total dietary fiber (TDF) has evolved since the 1970s, with methodologies constantly refined to align with updated definitions and analytical requirements. TDF data are integral to food composition databases and labeling. A summary of fiber components analyzed by various AOAC methods is illustrated in Figure 1.
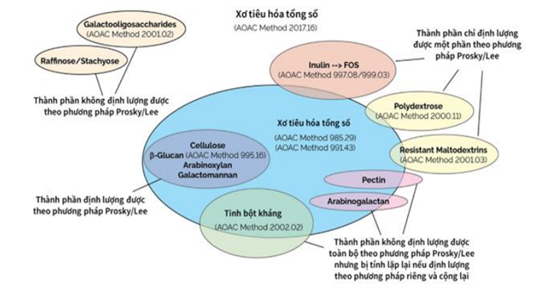
Figure 1. Dietary Fiber Components Analyzed Using Various AOAC Methods
- AOAC 985.29 and AOAC 991.43 (Prosky Methods)
Referred to as the "Prosky methods," these are classical approaches for determining TDF. AOAC 985.29 employs enzymatic hydrolysis with α-amylase, protease, and amyloglucosidase but does not capture low-molecular-weight dietary fibers (LMWDF) or most resistant starch types (except RS3). AOAC 991.43 extends this method to include soluble and insoluble fibers (AOAC 991.43 and AOAC 2001.03) and some non-digestible oligosaccharides.
- AOAC 2009.01 and AOAC 2011.25
These methods incorporate improved chromatographic conditions, ensuring precise quantification. AOAC 2011.25, endorsed globally by regulatory agencies such as Codex Alimentarius and the U.S. FDA, is the reference method for TDF analysis. Using α-amylase, protease, and amyloglucosidase, fibers are hydrolyzed at 37°C for 16 hours. Soluble fibers are quantified via high-performance liquid chromatography with refractive index detection (HPLC-RID), employing D-sorbitol as an internal standard and a Waters Sugar-Pak column. While capable of resolving some LMWDF, it cannot completely differentiate certain short-chain oligosaccharides, such as DP2 and DP3 of FOS and GOS.
- AOAC 2017.16 and AOAC 2022.01
These methods are designed to align with the latest definitions of dietary fiber. Enzymatic hydrolysis time is reduced from 16 hours to 4 hours, reflecting the average retention time of food in the small intestine during digestion. Sorbitol is replaced with glycerol as the internal standard, and dual TSK gel G2500PWXL columns enable the separation of DP2 and DP3 components of FOS and GOS. These methods provide comprehensive fiber profiling, including components aligned with Codex definitions.
With the goal of becoming one of the leading institutions in Vietnam and the region in the field of food testing and other related specialized areas, the National Institute for Food Control has been equipped with state-of-the-art facilities and a highly qualified team of professionals. NIFC continuously updates and develops the most advanced analytical techniques for dietary fibre to meet customer demands. Depending on the specific sample matrix, appropriate analytical techniques are selected. For natural products that do not contain added soluble fibres such as GOS, FOS, inulin, or polydextrose, NIFC employs AOAC 991.43 to determine total, soluble, and insoluble fibre content using the automated fibre analysis system from Ankom (Figure 2). For food products with declared fibre compositions, the corresponding specific analytical methods are utilized. For samples with unknown compositions, the institute prioritizes the latest method, AOAC 2022.01, which involves analysis on a high-performance anion-exchange chromatography system with pulsed amperometric detection (HPAEC-PAD) (Figure 3). This method simulates food hydrolysis conditions closely resembling digestion in the small intestine and comprehensively determines all fibre components as defined by Codex standards.
These methods have been validated in accordance with ISO 17025:2017 standards and certified by Vilas, ensuring accurate and reliable results with short analysis times and cost-effectiveness.
.jpg)
Figure 2. Automated System for Total, Soluble, and Insoluble Fiber Analysis (Ankom)

Figure 3. High-Performance Anion-Exchange Chromatography System with Pulsed A
amperometric Detection (HPAEC-PAD)
Author: Le Thi Thuy
National Institute of Food Control
Main Office:
Science and Technology Service Center - National Institute for Food Control
Address: 65 Phạm Thận Duật, Mai Dịch, Cầu Giấy, Hanoi
Hotline: 085 929 9595
Email: baogia@nifc.gov.vn / nhanmau@nifc.gov.vn
Representative Office in Ho Chi Minh City:
Address: Tan Cang - Cat Lai Port, Room A102, Gate B, Cat Lai Port, 1295B Nguyen Thi Dinh Street, Cat Lai Ward, Thu Duc City, Ho Chi Minh City
Phone: 028.37.400.888 / Hotline: 0918.959.678 (Mr. Nghị)
Email: baogia@nifc.gov.vn / vpsg.nifc@gmail.com
Representative Office in Hai Phong:
Address: No. 1 Ngo Quyen Street, Hai Phong City
Phone: 0225.8830316 / Hotline: 0983.300.226 (Ms. Thương)
Email: vphp@nifc.gov.vn
References
[1]. B.V.M.Cleary, N.Ames, J.Cox, et al, "Total dietary fiber (CODEX definition) in foods and food ingredients by a rapid enzymatic-gravimetric method and liquid chromatography: Collaborative study, first action 2017.16," Journal of AOAC International, 102(1), pp. 196-207, 2019.
[2]. Mudgil, D., & Barak, S. (2013). Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. International journal of biological macromolecules, 61, 1-6.
[3]. Khorasaniha, R., Olof, H., Voisin, A., Armstrong, K., Wine, E., Vasanthan, T., & Armstrong, H. (2023). Diversity of fibers in common foods: Key to advancing dietary research. Food Hydrocolloids, 139, 108495.
[4]. Lawlor, D. A., Smith, G. D., & Ebrahim, S. (2004). Hyperinsulinaemia and increased risk of breast cancer: findings from the British Women's Heart and Health Study. Cancer Causes & Control, 15, 267-275.